As a metabolite, the Tetrahydrocurcuminoids (THCs) have a distinct advantage of having higher bioavailability than the parent curcuminoids. Mechanistically the parent curcuminoids once ingested reach to intestine, where they need to pass through the absorption barrier to enter the biological system. Further, the reductase system at the cellular level then converts the curcumin to Tetrahydrocurcumin (THC). Investigations have revealed that the THC is an active metabolite of the curcumin.
In order to study, the distribution of the curcumin and its metabolite THC in the biological system, Okada et al., in 2001 performed an animal study involving feeding a diet containing 0.5% of curcumin and 0.5% of THC to two different groups of mice for 1 month.
The results showed that the mice fed with 0.5% of curcumin in diet showed only a small amount of curcumin and its conjugate in the serum, whereas curcumin was undetected in the liver tissue. Whilst, the mice fed with 0.5% of THC in diet showed not only higher concentration of THC and its conjugate in the serum but also in the liver tissue.
Distribution of curcuminoids in liver and serum of mice fed with curcumin
| Liver | Blood serum | |||
|---|---|---|---|---|
| Free (nmol/mg) |
Conjugate (nmol/mg) |
Free + Conjugate (nmol/mL) |
||
| Curcumin | Not detected |
Not detected |
0.6 ± 0.1 | |
| THC | Not detected |
3.5 ± 0.4 | 14.4 ± 3.9 | |
Distribution of curcuminoids in liver and serum of mice fed with THC
| Liver | Blood serum | |||
|---|---|---|---|---|
| Free (nmol/mg) |
Conjugate (nmol/mg) |
Free + Conjugate (nmol/mL) |
||
| Curcumin | Not detected |
Not detected |
Not detected |
|
| THC | 2.5 ± 0.6 | 7.9 ± 1.6 | 43.4 ± 15.5 | |
This is in agreement to a later finding by Hassaninasab et al., (2011) who discovered the curcumin converting enzyme from commensal microbe E. coli in the human gut. The study found that purified “NADPH-dependent Curcumin/Dihydrocurcumin reductase” (CurA) enzyme from E. coli strain K-12, sub strain DH10B catalyzed two reaction steps, wherein curcumin was first reduced to Dihydrocurcumin (DHC) followed by the latter’s sequential reduction to THC. Thus, the study led to the discovery of two-step curcumin reductive metabolic pathway catalyzed by CurA. The products DHC and THC were generated from curcumin through reductive destruction of the chromatic diarylheptatrienone chain. The lack of production of further reduced reaction products suggested that CurA catalyzes reduction of only compounds with C-C double bonds. The CurA was found to be substrate-specific, NADPH dependent, metal independent and stable at wide pH range (4.5–12.0). All these evidences show that CurA could be a potent catalyst for the production of large amount of value-added product in vitro.
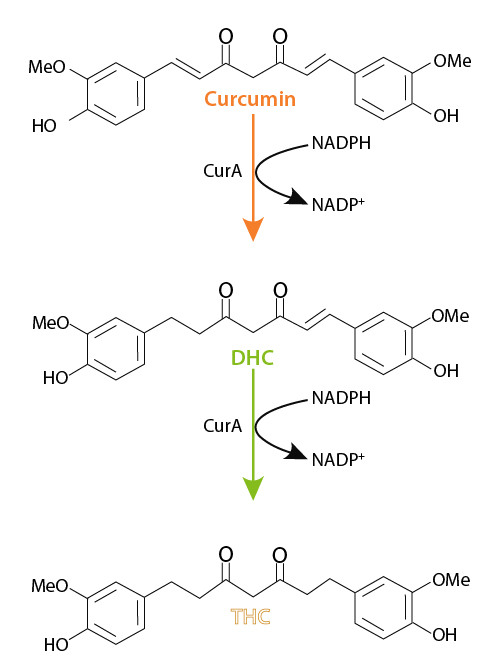
(Adapted from Hassaninasab et al., 2011)
The safety and pharmacokinetics of curcumin and its metabolite, THC, were studied in Beagle dogs following intravenous infusion of Lipocurc™ (liposomal curcumin) at a dose of 10 mg/kg over a 2 h and 8 h infusion times (Helson et al., 2012; Matabudul et al., 2012). Infusion of Lipocurc™ in dogs at two different time periods resulted in higher plasma levels of curcumin and THC with a 2 h infusion compared to an 8 h infusion. The plasma levels of THC were 6.3–9.6 fold higher than curcumin during both infusion rates, suggesting the putative presence of a high-rate of curcumin reducing enzyme in blood or tissues and comparatively slower rate of blood THC clearance. The plasma half-lives of both compounds following 2 h infusion ranged from 0.4–0.7 h and were a consequence of both hepatic and renal clearance.
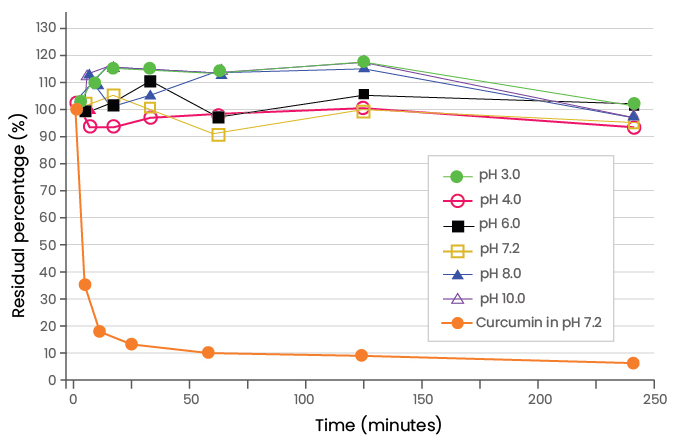
(Adapted from Pan et al., 1999, drug metabolism and disposition)
The safety and pharmacokinetics of curcumin and its metabolite, THC, were studied in Beagle dogs following intravenous infusion of Lipocurc™ (liposomal curcumin) at a dose of 10 mg/kg over a 2 h and 8 h infusion times (Helson et al., 2012; Matabudul et al., 2012). Infusion of Lipocurc™ in dogs at two different time periods resulted in higher plasma levels of curcumin and THC with a 2 h infusion compared to an 8 h infusion. The plasma levels of THC were 6.3–9.6 fold higher than curcumin during both infusion rates, suggesting the putative presence of a high-rate of curcumin reducing enzyme in blood or tissues and comparatively slower rate of blood THC clearance. The plasma half-lives of both compounds following 2 h infusion ranged from 0.4–0.7 h and were a consequence of both hepatic and renal clearance.
